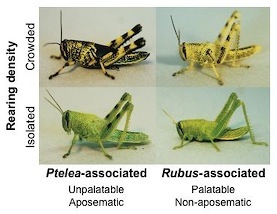
 These are examples of the differential expression of density-dependent colour polyphenism between different host plant-associated forms of Schistocerca lineata grasshoppers. Juveniles from Ptelea trifoliata-associated populations are unpalatable to vertebrate predators by virtue of feeding on their host plant and express density-dependent warning colouration (aposematism). Closely related, but genetically distinct nymphs from Rubus trivialis-associated populations do not derive unpalatability from their host plant. They should not benefit from the expression of density-dependent warning coloration, and accordingly their ability to express density-dependent colour change has been much reduced by natural selection. After Sword (2002).
These are examples of the differential expression of density-dependent colour polyphenism between different host plant-associated forms of Schistocerca lineata grasshoppers. Juveniles from Ptelea trifoliata-associated populations are unpalatable to vertebrate predators by virtue of feeding on their host plant and express density-dependent warning colouration (aposematism). Closely related, but genetically distinct nymphs from Rubus trivialis-associated populations do not derive unpalatability from their host plant. They should not benefit from the expression of density-dependent warning coloration, and accordingly their ability to express density-dependent colour change has been much reduced by natural selection. After Sword (2002).
Understanding patterns of resource use can have far reaching implications. A good example is my discovery of the first case of density-dependent warning coloration found in nature (Sword, 1999). In a series of laboratory experiments, I showed that juvenile S. lineata (emarginata) grasshoppers express density-dependent phenotypic plasticity in coloration whereby they are commonly green when reared at low population density, but become yellow-and-black when reared at high density. I then demonstrated that the grasshoppers derive gut-content mediated toxicity to predators simply by consuming their primary host plant. By linking host plant use to the expression of density-dependent color change, I demonstrated that the grasshoppers exhibit a shift in anti-predator strategy from crypsis at low density to warning coloration at high density in experiments using visually-hunting vertebrate predators.
This finding led me to propose a new hypothesis for the evolution of warning coloration, a debate dating back to discussions between Darwin and Wallace, whereby plasticity in coloration can act as an adaptive intermediate stage (Sword, 1999). My collaborators and I went on to show that S. lineata populations utilizing different plants in the field constitute genetically-distinct host plant-associated mtDNA lineages (Dopman et al., 2002). These genetically-distinct lineages differ in the toxicity to their predators that they derive from their respective host plants. As a result, their reaction norms for the expression of density-dependent color change have differentially evolved in accordance with the expectations of aposematic theory (Sword, 2002; see graphic).

Relevant publications
Simpson, S.J. & Sword, G.A. (2009) Phase polyphenism in locusts: Mechanisms, population consequences, adaptive significance and evolution. In: Whitman, D. & Ananthakrishnan, T. (eds.) Phenotypic Plasticity of Insects: Mechanisms and Consequences. Science Publishers Inc., Plymouth, pp 147-190.
Sword, G.A. (2002) A role for phenotypic plasticity in the evolution of aposematism. Proceedings of the Royal Society of London B 269:1639-1644.
Dopman, E.B., Sword, G.A., & Hillis, D.M. (2002) The importance of the ontogenetic niche in resource-associated divergence: evidence from a generalist grasshopper. Evolution 56(4):731-740. (Cover photo)
Sword, G.A. (2001) Tasty on the outside, but toxic in the middle: Grasshopper regurgitation and host plant-mediated toxicity to a vertebrate predator. Oecologia 128:416-421.
Sword, G.A., Simpson, S.J., El Hadi, O.T.M. & Wilps, H. (2000) Density-dependent aposematism in the Desert locust. Proceedings of the Royal Society of London B 267:63-68.
Sword, G.A. & Simpson, S.J. (2000) Is there an intraspecific function for density-dependent color change in the Desert locust? Animal Behaviour 59: 861-870.
Sword, G.A. (1999) Density-dependent warning coloration. Nature 397:217.
Sword, G.A. & Dopman, E.B. (1999) Developmental specialization and geographic structure of host plant use in a polyphagous grasshopper, Schistocerca emarginata (=lineata) (Orthoptera: Acrididae). Oecologia 120:437-445.